
The Evidence Layer
for AI
Governance committees don't trust policy documents. They trust evidence. We help AI vendors generate proof their controls actually ran.


The Problem
Your healthcare AI is stuck in procurement
You've built AI that works. But every health system governance committee asks questions you can't answer:
How do we know your guardrails actually ran on every inference?
Can you prove consent was captured before each AI interaction?
What happens when the plaintiff's attorney asks for evidence?
Your dashboard says "compliant."
Their lawyers say "prove it."



What's Happening Now
The trust gap is real—and growing
~100,000 patients. Allegation: ambient scribe hallucinated consent. The AI vendor can't cryptographically prove otherwise.
AI Governance Committee blocked vendor contract over data ownership and auditability concerns. Vendor's dashboard wasn't enough.
For AI vendors: Every month without provable controls is another deal stuck in legal review, another competitor who might figure this out first.

What We Do
Receipts, not promises
We generate cryptographically signed proof that your safety controls executed at inference time. Timestamped. Independently verifiable. Mapped to the frameworks procurement committees recognize.
When the attorney asks "prove it"—you can. When the procurement committee asks for evidence—you have it. When the auditor arrives—you're ready. And after the deal closes, you have ongoing proof for internal compliance, board reporting, and liability protection.



Why Now
The vendor who proves execution closes first
Regulations create deadlines. But the real urgency is competitive: while your deals are stuck in legal, someone else is figuring this out.
COMPETITIVE ADVANTAGE
Close faster: Answer security questionnaires definitively
Reduce friction: Give governance committees what they need
Differentiate: Your competitors can't prove execution. You can.
REGULATORY DEADLINES
Standard of care precedes law. NIST AI RMF and ISO 42001 are already the de facto duty of care.
The window: In 18 months, AI vendors without cryptographic proof will lose deals to those who have it.
The Market
Healthcare AI is exploding. Governance isn't keeping up.
TAM — Healthcare AI market
SAM — AI Governance Software
SOM — Healthcare AI Governance
Initial wedge: ambient AI vendors
Texas AG settled with Pieces Tech over AI hallucination claims (Sept 2024)
The governance gap
of health orgs have AI governance
Yet 78% face regulatory scrutiny
of AI pilots fail
Governance gaps, not tech problems
FDA-authorized AI devices
Up from 950 in 2024 — accelerating
Sources: MarketsandMarkets, Grand View Research, FDA, Advisory Board, Texas AG

The Insight
No one else proves execution
Guardrails block bad things. GRC platforms document policies. Neither proves your controls actually ran on every inference.

Infrastructure Players
Cloudflare, AWS, Alinia
Block bad things at runtime
Gap: No compliance mapping. Logs ≠ proof.
GRC Platforms
Vanta, Credo AI
Document policies and procedures
Gap: No runtime enforcement. Trust us.
GLACIS
Cryptographic Attestation
Prove execution at inference time
Runtime + Compliance + Proof
How It Works
Drop-in. Zero data egress. Sub-50ms.
Your Healthcare AI
Gen AI in clinical workflows
GLACIS
SIDECARAttest → Hash → Sign
<50ms • 0 bytes PHI out
Witness Network
Hashes only
Merkle anchor
Zero-Egress = No BAA
No PHI leaves customer boundary. Eliminates months of legal review.
Third-Party Verifiable
Ed25519 signatures + RFC 6962 Merkle trees. Independent verification.
Drop-In Deployment
Lambda layer or container sidecar. Works with any LLM provider.

The Platform
Live. Shipping. Production-Ready.
Live at app.glacis.io — governance platform, attestation feed, and witness network operational.

Cryptographic Attestations
Merkle-anchored receipts with HIPAA identifiers
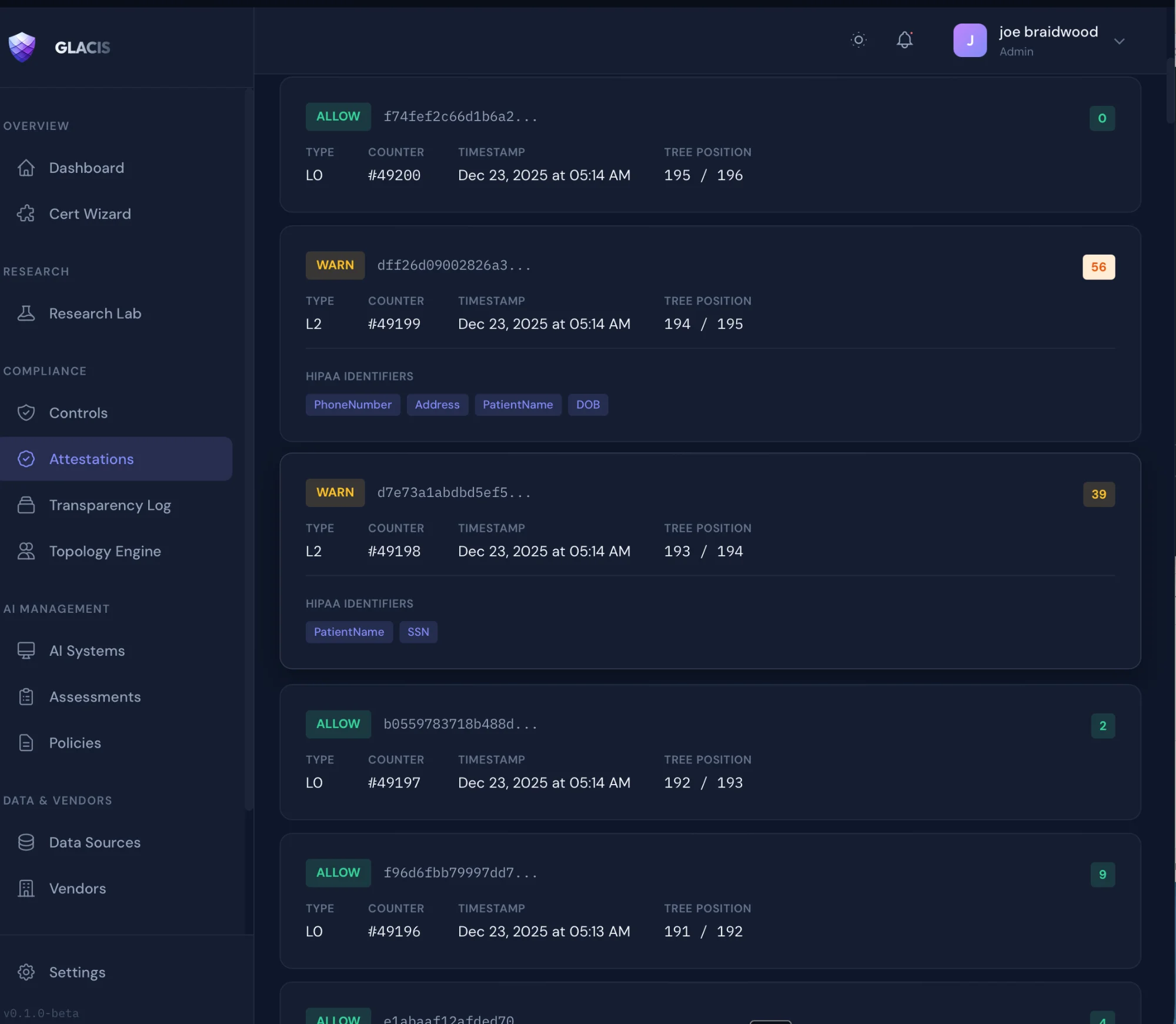
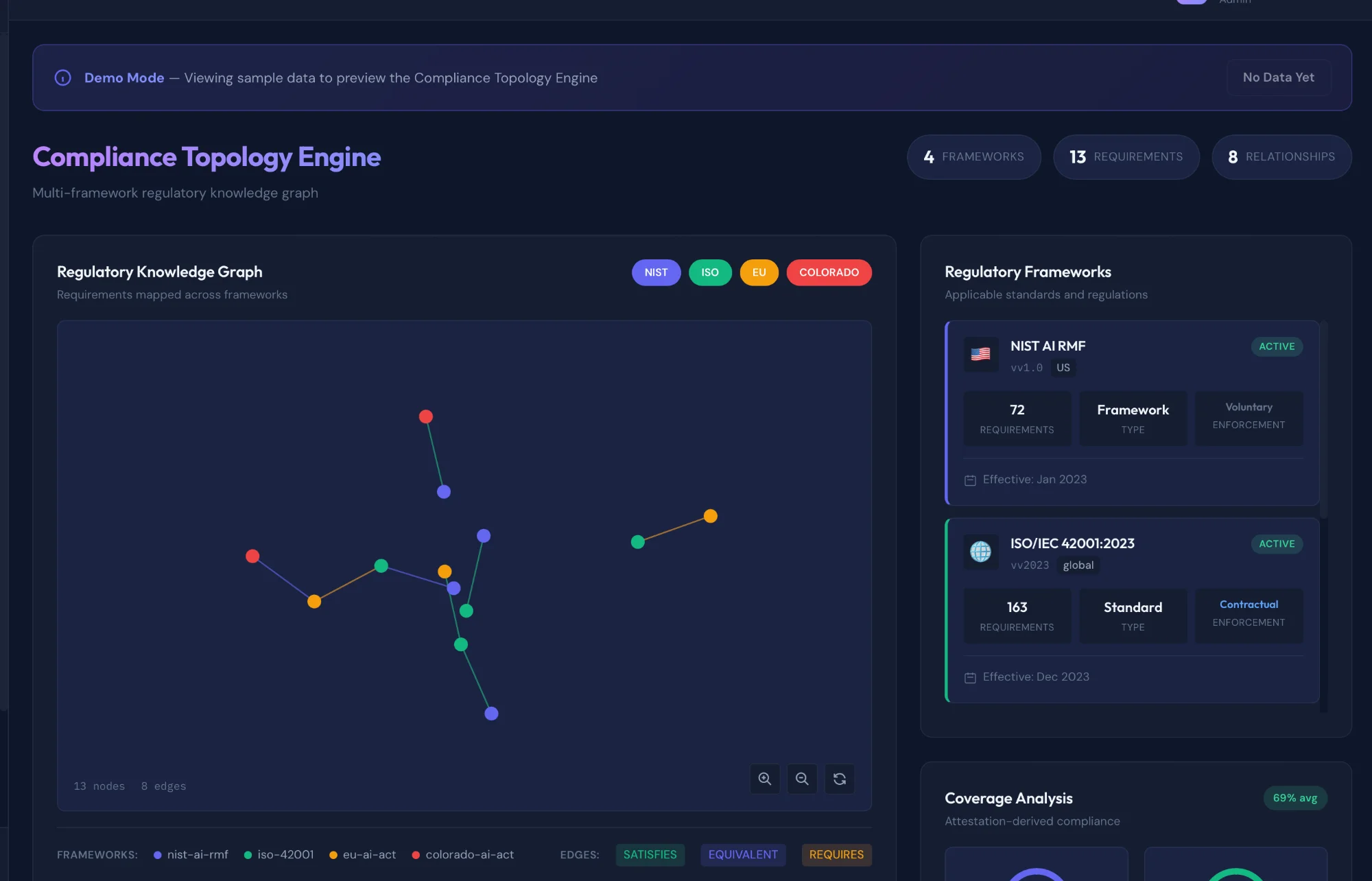
Governance Topology Engine
NIST AI RMF • ISO 42001 • EU AI Act • Colorado
Traction
Early traction. Momentum building.
Next milestone
JPM Healthcare 2025
50k+
visits/month
Colorado-based. Facing Colorado AI Act deadline.
Consent attestation • PHI proof • Guardrail evidence
In pipeline

Inbound interest
Health Systems
AI governance inquiries
AI Vendors
Procurement pain resonates
Investors
Healthcare + AI governance thesis
Team
FDA Authorized. Enterprise Deployed.
We've lived this problem.

Joe Braidwood
Co-Founder & CEO
SwiftKey → 1 in 4 smartphone users
Founding exec, $250M Microsoft exit. Scaled AI to hundreds of millions of users. Cambridge Law.

Dr. Jennifer Shannon
Co-Founder & Chief Medical Officer
Cognoa → First FDA De Novo for AI diagnostics
Medical Director at Cognoa. Navigated FDA authorization for AI that diagnoses autism in children. Knows the regulatory path.

Caer
Rust & Cryptography Lead
10+ years production • WPI

Atreya
AI Engineer
Model eval & red teaming • WPI • Ex-PAAI
Advisors

70+
Patent claims filed
4 families • Fenwick & West

Defensibility
Building the Category Definition
70+
Patent claims filed
4 families • Fenwick & West
Co-Epoch Attestation
Binds receipts to specific binary versions. Prevents "binary substitution" attacks.
Statistical Safety Signals
Mathematically rigorous sampling for continuous compliance verification.
Zero-Payload Egress
Cryptographic commitments without data exposure. PHI never leaves boundary.
Federated Witness Network
Distributed attestation for independent verification at scale.
Filed Nov 2025 — First-mover advantage in attestation-based AI compliance.
The Ask
$2M to own healthcare AI proof
Use of Funds
2 senior engineers (Rust, infra)
Health system sales, design partners
Legal, compliance, infrastructure
18-Month Milestones
5+ paying design partners
Ambient AI vendors in production
$500K ARR
Platform + Evidence Pack revenue
First health system direct
Enterprise governance platform
Series A ready
Metrics for $8-12M raise
Why now: First-mover window before Colorado (June 2026) and EU AI Act (Aug 2026) create compliance scramble
Let’s Talk
Let’s connect
We're looking for healthcare AI teams who need to prove their safety controls work—and want to be the first to do it with cryptographic proof.
DESIGN PARTNERS WE'RE LOOKING FOR
→ LLMs or agentic AI in clinical workflows
→ Safety guardrails that need evidence they ran
→ Pursuing FDA pathways or regulatory authorization
→ Selling to health systems with governance committees


The Evidence Layer for AI
